Patient stem cells are a powerful tool for understanding disease and testing therapies, since they can be converted into the specific cell types affected by disease — whether it be a neuron or kidney cell. At the Harvard Stem Cell Institute (HSCI), our researchers are pushing the boundaries of how we can take advantage of these disease models to understand mechanisms and find potential therapeutics.
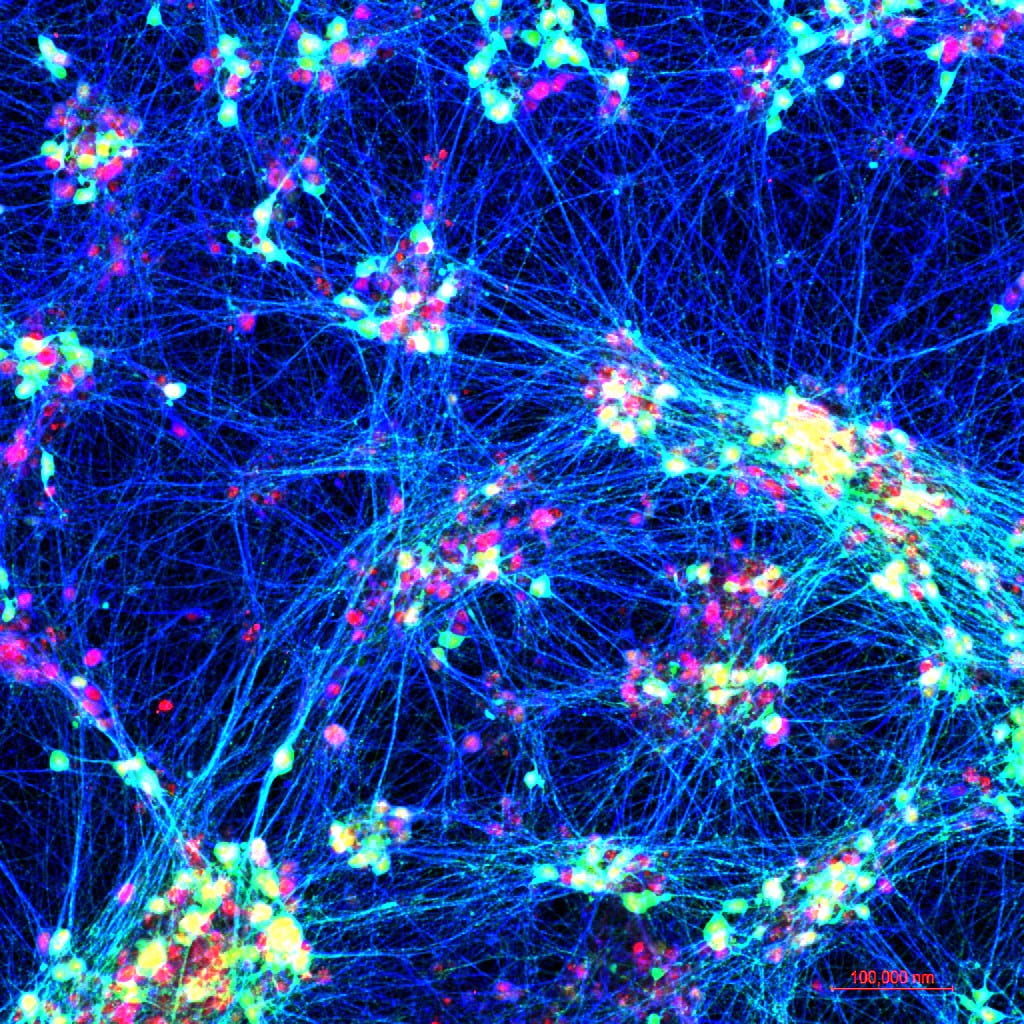
Patient-specific models of Alzheimer’s disease
Alzheimer’s disease progresses differently across patients in terms of underlying cause, genetic makeup, age at onset, and disease course, among other factors. To model how an individual’s unique genetic background influences disease development, a large set of stem-cell-derived neurons was created by HSCI researchers led by Tracy Young-Pearse, Ph.D., Co-Leader of the Nervous System Diseases Program and Principal Faculty member.
The researchers studied a group of 53 individuals — with and without Alzheimer’s disease — that had multiple types of data available, including long-term clinical measures of cognitive decline and genomic sequencing. The researchers created stem cells from each individual and turned them into neurons. The specific types of amyloid beta protein and tau protein found in the neurons predicted whether the individual developed the disease, as well as the rate of cognitive decline. Additionally, the researchers found a specific molecular pathway that linked the two proteins’ behavior.
This collection of neurons is a powerful way to model the complexity of Alzheimer’s disease, opening the door to understanding an individual’s risk of developing the disease and which specific drugs are most effective.
Image: Neurons made from human stem cells, used to study Alzheimer’s disease development. Credit: Young-Pearse Lab, Brigham and Women’s Hospital
This is the first time we have a system in place to study living human brain cells from many people to understand better why some develop Alzheimer’s disease in a very specific way and others are resistant to the disease.
Tracy Young-Pearse, Ph.D.
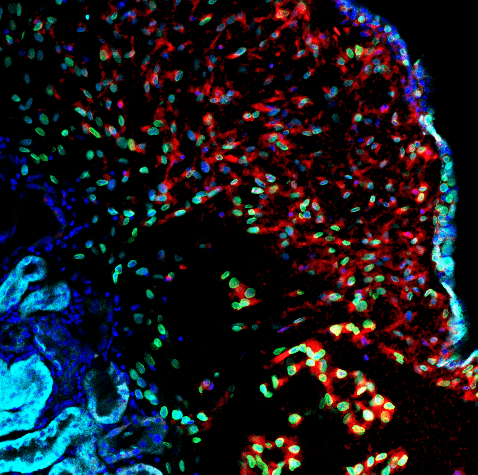
An organoid model of kidney tumors
In the genetic disease Tuberous Sclerosis Complex, most patients develop a tumor called renal angiomyolipoma (AML), which can lead to kidney failure and premature death. Researchers’ understanding of AML has been limited by a lack of lab models, since animals with the necessary genetic mutations largely die before birth. HSCI Affiliate Faculty member Dario Lemos, Ph.D., took a new approach to solve this problem.
The researchers started with patient stem cells, genetically edited them, and directed them to become kidney cells. The cells showed characteristics of AML and formed organoids, or 3D miniature organs, in a lab dish. The researchers transplanted the organoids into rats, successfully using the resulting model to find a mechanism of tumor resistance and to treat tumors in a targeted way. This organoid transplantation model is an innovative way to study disease mechanisms and develop potential therapies.
Image: A kidney organoid model of a tumor, made using patient stem cells. Credit: Lemos Lab, Brigham and Women’s Hospital
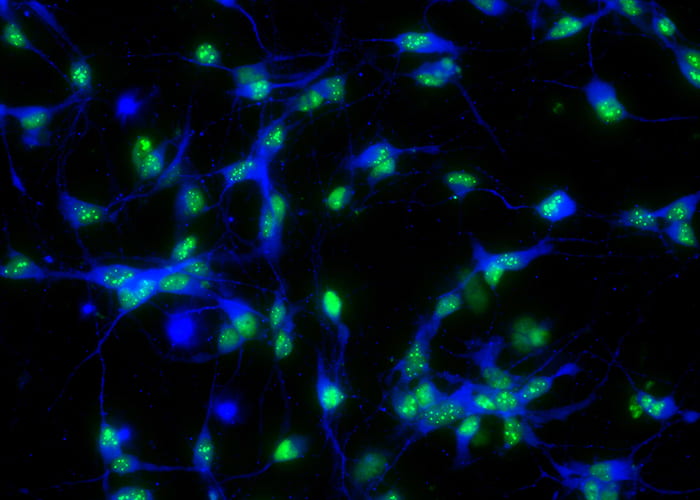
A high-throughput platform to discover ALS drugs
HSCI Principal Faculty member Clifford Woolf, M.B., B.Ch., Ph.D., developed a high-throughput platform for discovering drug targets to treat amyotrophic lateral sclerosis (ALS). The researchers used stem cells derived from patients with ALS to create motor neurons, the cell type affected in ALS. Advanced imaging technology was used to measure whether drug candidates reduced the neurons’ hyperexcitability, or tendency to fire excessively.
After screening a library of 2,900 compounds, the researchers confirmed two known ALS drug targets and identified a new one. A drug for one of the targets is currently under clinical development by QurAlis, a biotechnology company founded by Woolf and former HSCI Principal Faculty member Kevin Eggan. Additionally, their approach of using patient-derived cell models to screen for drugs can be applied to many other diseases.
Image: Neurons created from ALS patient stem cells. Credit: Woolf Lab, Boston Children’s Hospital