Developing new therapies requires a foundational understanding of how the body works, including both healthy and disease processes. Harvard Stem Cell Institute (HSCI) researchers are investigating these biological pathways in fine detail, following the directions of molecules as they move within and in between cells, with the ultimate goal of therapeutic intervention.
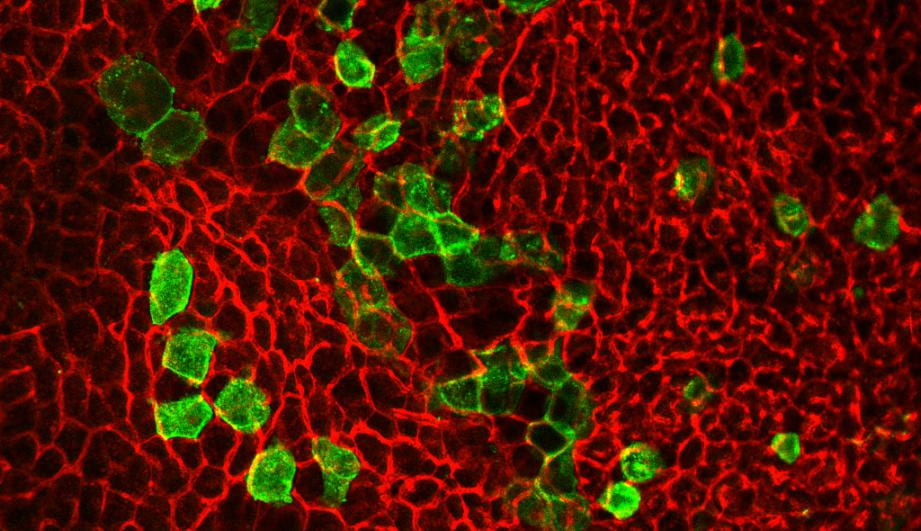
A new source of energy-burning fat cells
Brown fat cells direct the process of thermogenesis, becoming activated in cold temperatures to burn calories and generate heat. As a result, they are a potential target for treating obesity. Researchers led by HSCI Principal Faculty member Yu-Hua Tseng, Ph.D., identified a new source of these energy-burning brown fat cells.
The researchers used single-cell RNA sequencing to study brown fat from mice housed at different temperatures and lengths of time. They found that smooth muscle cells expressing Trpv1 — a receptor protein that senses noxious stimuli, including pain and temperature — gave rise to brown fat cells. Trpv1 can be potentially targeted to increase the number of brown fat cells, a strategy to treat obesity and other metabolic disorders.
Image: Brown fat cells derived from cells expressing the Trpv1 receptor protein (in green), in mice exposed to cold temperature. Credit: Tseng Lab, Joslin Diabetes Center
A direct control system for blood production
Researchers led by HSCI Co-Director David Scadden, M.D., discovered a new way that the immune system responds to stress such as infection. They showed for the first time in animals that in the bone marrow, bone-forming cells released RNA that is packaged in vesicles, or small membrane-enclosed particles. The vesicles were taken up by blood stem cells, which in turn ramped up the production of immune cells. This process was more direct than the conventional system of activating a complex hormone-signaling pathway, and is a new way of thinking about how the body rapidly reacts to infection.
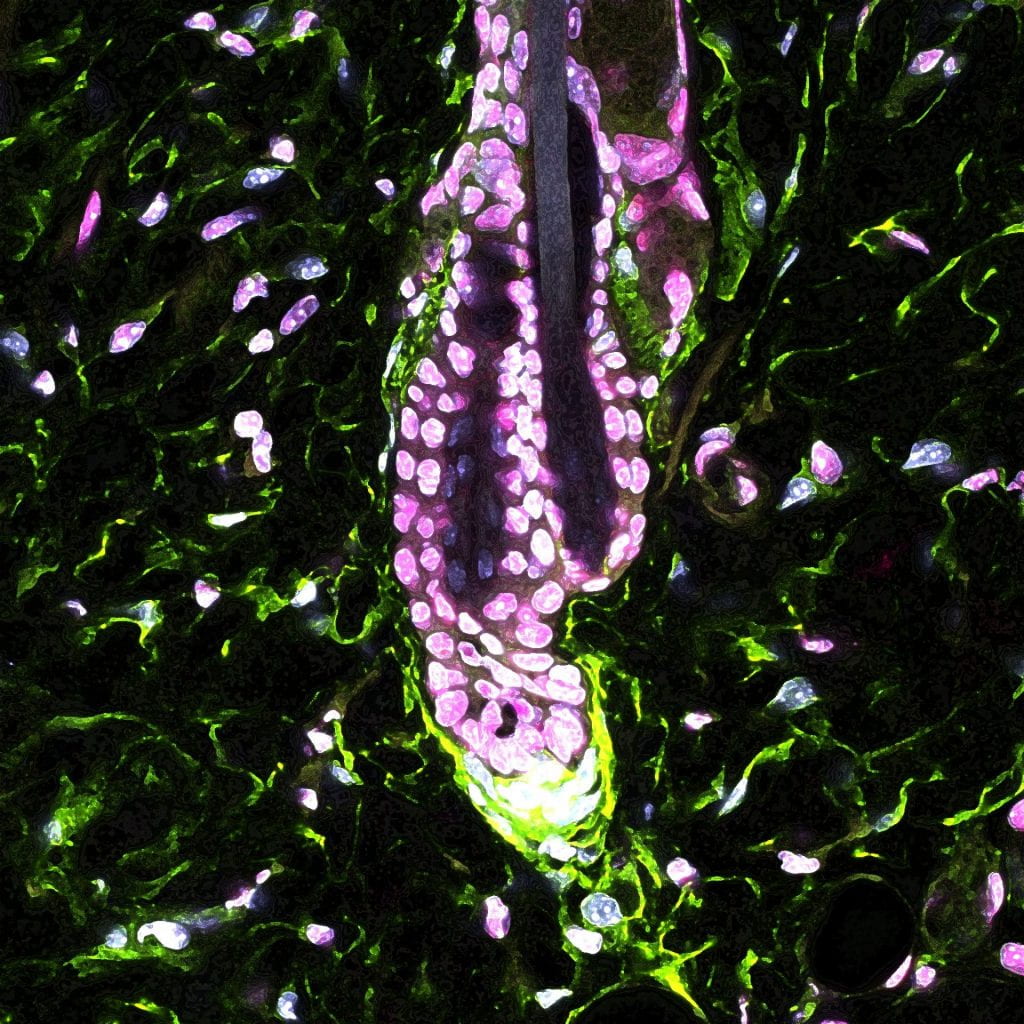
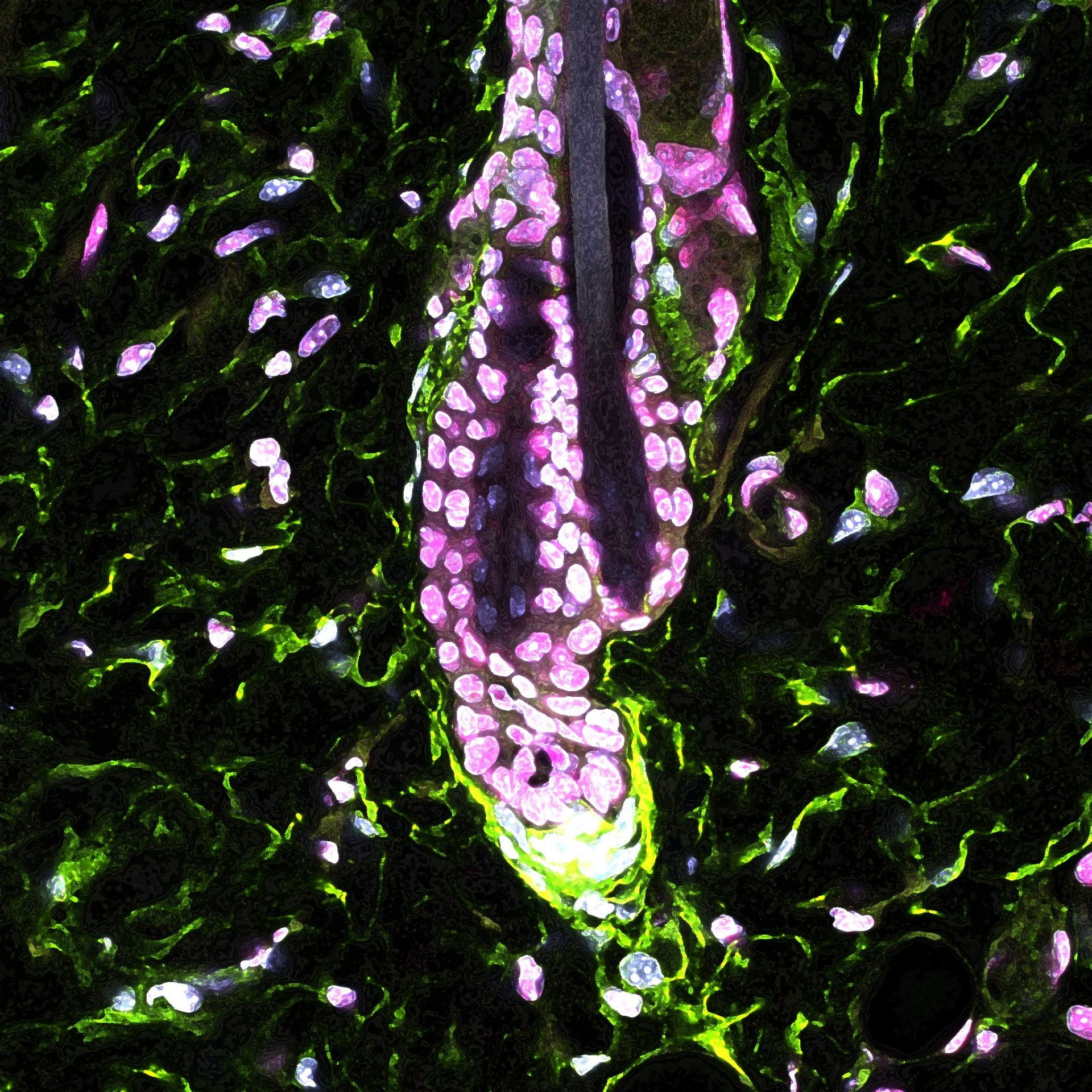
How chronic stress leads to hair loss
HSCI Principal Faculty member Ya-Chieh Hsu, Ph.D., identified the biological mechanism of how chronic stress leads to hair loss, confirming long-standing observations that the two are connected.
The researchers used a mouse model of chronic stress to study hair follicle stem cells, which regenerate the hair follicle and hair. They found that the stress hormone corticosterone directed hair follicle stem cells to stay in an extended resting phase without regenerating tissue. The stress signal was first received by dermal cells surrounding the hair follicle, preventing them from releasing Gas6, a molecule that activates stem cells. When researchers added back Gas6, stem cells could regenerate hair even under stress.
The Gas6 pathway is a potential target for promoting hair growth. More broadly, researchers now have a better understanding of how stress plays a role in stem cell biology and cross-organ signaling.
Image: Underneath the hair follicle, dermal papilla cells (green) produce the Gas6 molecule that activates hair follicle stem cells. Credit: Hsu Lab, Harvard University
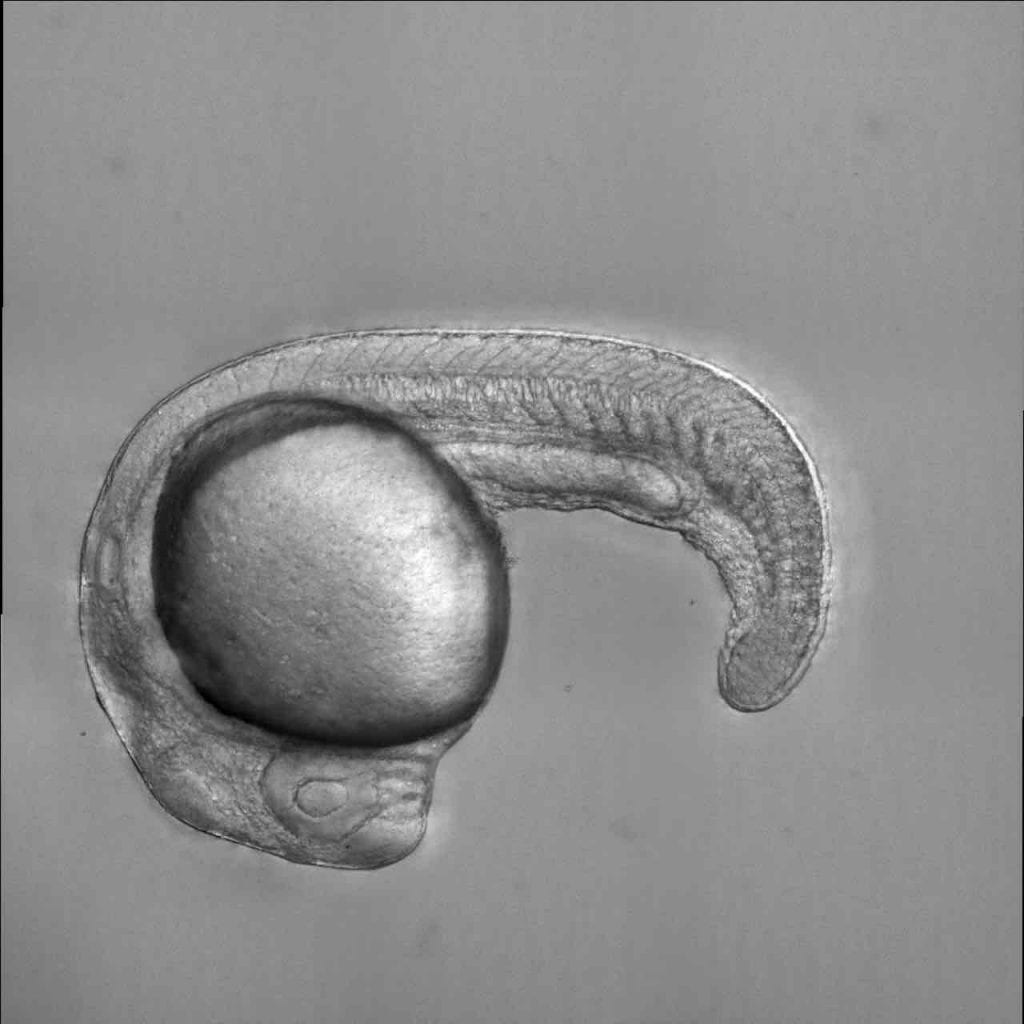
Metabolism’s role during red blood cell development
Each tissue in the body has different requirements for metabolism, or how it uses energy to function. For example, a muscle needs different molecules to fuel a contraction compared to a pancreas that produces insulin. Even though metabolic pathways may be the same across tissues, it is not fully understood how each tissue directs which one to predominantly use for its own specific needs.
Researchers led by HSCI Executive Committee Chair Leonard Zon, M.D., found a new connection between metabolism and red blood cell development. The researchers studied a zebrafish model of anemia, which was defective in producing red blood cells because it lacked a specific DNA-binding protein. They identified the detailed mechanism for how red blood cell precursors use the DNA-binding protein to control metabolism in the mitochondria. This pathway can be potentially targeted in diseases such as anemia to restore red blood cell production, as well as certain types of cancer.
Image: Zebrafish embryo that models anemia, containing a defective DNA-binding protein that leads to an inability to produce red blood cells. Credit: Zon Lab, Harvard University