Harvard Stem Cell Institute (HSCI) researchers push toward our ultimate goal of using stem cell biology and regenerative medicine to cure disease. Working across the spectrum of human disease, our researchers are creating next-generation therapies by developing cell transplants, coaxing existing cells to regenerate, and taking the first steps toward growing replacement organs.
The first clinical trial results for Type 1 diabetes cell transplants
In Type 1 diabetes, the immune system attacks beta cells in the pancreas, so that they can no longer produce the insulin necessary to control blood sugar. Over the last 15 years, HSCI Co-Director Douglas Melton, Ph.D., pioneered the process of directing stem cells to become functional beta cells, which can be then transplanted into patients. To bring this therapy to the clinic, Melton previously launched the startup company Semma Therapeutics, which was later acquired by Vertex Pharmaceuticals.
In 2021, Vertex announced the initial results of their clinical trial: the first patient to receive a stem-cell-derived beta cell transplant could successfully produce insulin and control blood sugar, greatly reducing the need for the usual insulin injections. The next step of developing this therapy will be to control the immune system’s reaction, so that patients can receive cell transplants without having to take immunosuppressants.
New multi-institutional center for Type 1 diabetes research
Douglas Melton is directing a new multi-institutional center to advance the next stage of Type 1 diabetes research. At the JDRF Center of Excellence in New England, researchers use stem cell and gene editing approaches to investigate why immune rejection happens and to create beta cells that can withstand it. The center is a collaboration with HSCI Principal Faculty member Stephan Kissler, Ph.D., at the Joslin Diabetes Center, as well as scientists at JDRF, UMass Chan Medical School, and the Jackson Laboratory.
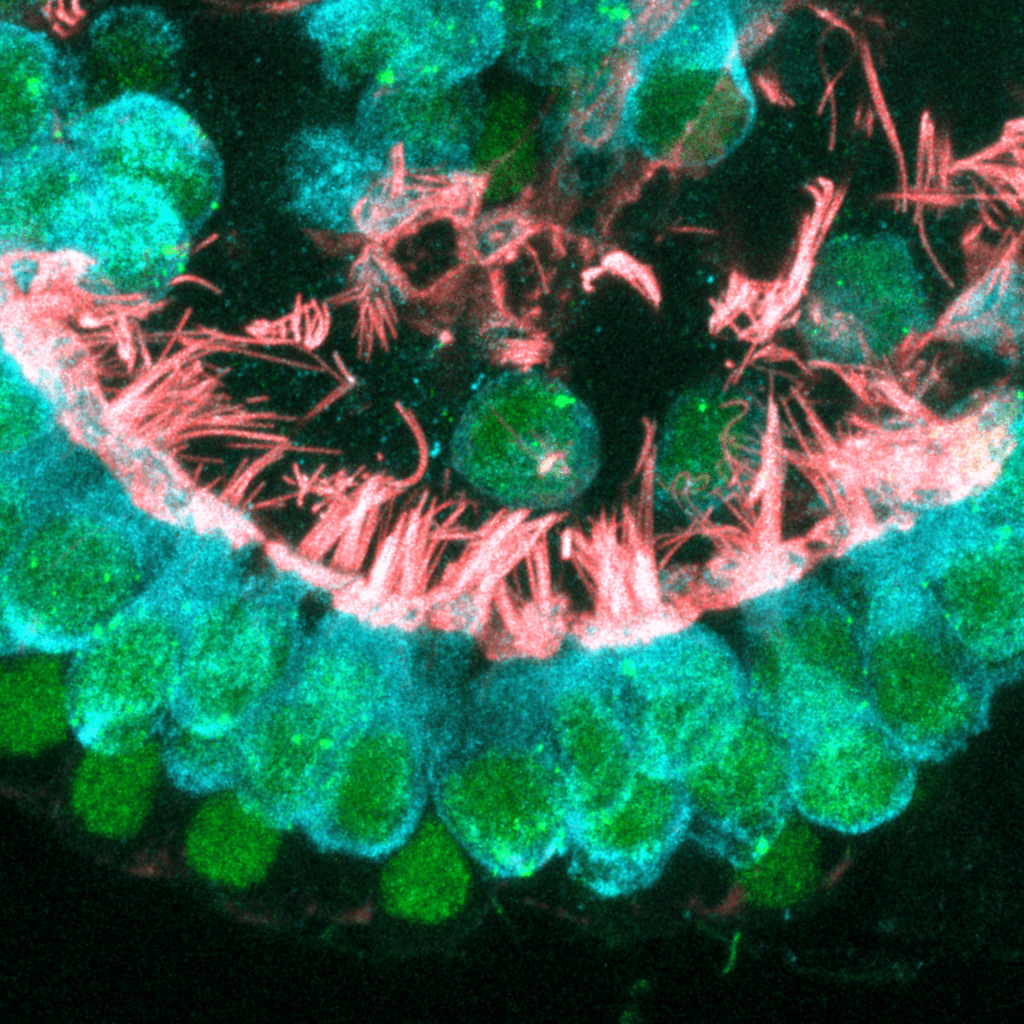
Pathways for preventing and rescuing hearing loss
HSCI Principal Faculty member Albert Edge, Ph.D., identified the biological pathway that can lead to deafness in Norrie disease, a rare condition caused by mutations in the NDP gene. The researchers studied a mouse model of Norrie disease that lacked the NDP gene. Without NDP, the hair cells of the inner ear died over time, leading to progressive deafness.
The researchers were able to restore hearing loss using two methods: 1) by stimulating a pathway previously found to be important for hair cell regeneration, and 2) by expressing NDP in cells located next to the hair cells. These approaches direct hair cells to recover their function, and are potential treatments to rescue hearing in both Norrie disease and other forms of hearing loss that are caused by hair cell death.
Image: Hair cells of the inner ear, which are affected in patients with Norrie disease. Credit: Edge Lab, Massachusetts Eye and Ear
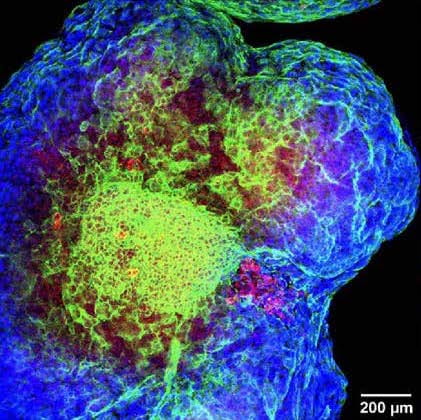
Generating cells that direct heart development in the embryo
During embryonic development, a type of cell called the “pre-epicardial cell” (PEC) helps to direct heart formation. Previously, researchers were able to create heart muscle cells in a lab dish, but not PECs. HSCI Principal Faculty member Harald Ott, M.D., identified a way to convert human stem cells into PECs.
When the PECs were grown together with heart muscle cells, both cell types matured and created a beating structure, both in 2D and — excitingly — in 3D cultures. This advance is an important step toward generating a tissue with the complex structure of the heart, which is a potential therapy for heart failure.
Image: When grown together in a lab dish, heart muscle cells and pre-epicardial cells organize into a complex structure. Credit: Ott Lab, Massachusetts General Hospital